Aim/Introduction
Up to one third of diffuse large B-cell lymphoma (DLBCL) patients experience relapse or fail to achieve complete remission during first-line treatment. Identification of poor prognosis patients might be further improved by radiomics. Radiomics analysis of imaging data provides quantitative features of tumor characteristics such as intensity, shape, volume, texture and intra- and inter-lesion heterogeneity. The objective of this study was to assess the added value of baseline quantitative radiomics features in DLBCL patients compared to currently used clinical characteristics such as the IPI score.
Materials and Methods
317 newly diagnosed DLBCL patients with baseline 18F-FDG PET/CT scans from the HOVON84 trial (Lugtenburg et al, JCO 2020) were included. Lesions were delineated using a fully automated preselection of 18F-FDG avid structures defined by a SUV ≥ 4.0 and volume >3mL. Missed lesions were added and non-tumour regions were removed (accurate tool, https://petralymphoma.org). Next, 490 radiomics features were extracted from the volume of interest using RaCat software (Pfaehler et al, 2019). To reduce feature space dimensions, we made a preselection of clinically most relevant radiomics features based on literature (SUVmax, SUVmean, SUVpeak, total lesion glycolysis, metabolic tumor volume (MTV), dissemination features and sphericity) and used logistic regression with backward feature selection to predict 2-year time to progression (TTP), defined as time from baseline PET/CT to progression. Patients who died without progression were censored at date of death. Furthermore, we tested the predictive value of known clinical predictors (age (cut off: >60 years), WHO performance status (cut-off: ≥ 1 and ≥2), Ann Arbor stage, extranodal involvement (cut-off: ≥ 1 and >1), lactate dehydrogenase (LDH) level and bulky disease (≥ 10 cm)) and of a model that combined radiomics and clinical parameters. Model performance was assessed using repeated cross-validation (5 folds, 2000 repeats) yielding the mean receiver-operator-characteristics curve integral (AUC). High- and low-risk groups were defined based on prevalence of events, diagnostic performance was assessed using positive- and negative predictive values. Patients censored before 2 years of follow-up were excluded for the prediction models and diagnostic performance.
Results
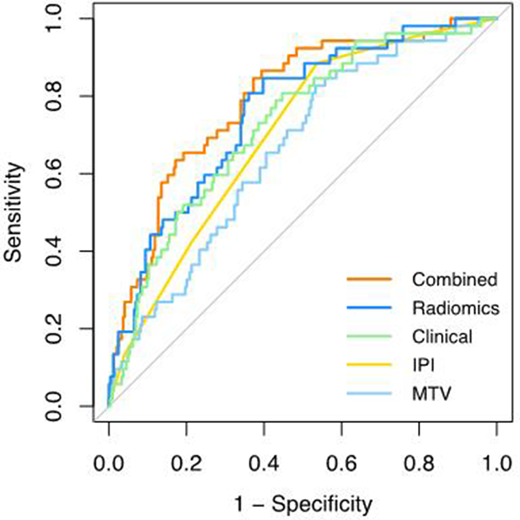
The categorical IPI score yielded in a cross-validated AUC (CV-AUC) of 0.68 (Figure 1). The highest performance for the radiomics model was observed for the natural logarithms of MTV and SUVpeak and the maximal distance between the largest lesion and any other lesion (Dmaxbulk), which yielded in a cross-validated AUC (CV-AUC) of 0.75±0.07, which was significantly higher than the discriminative power of the MTV model (CV-AUC: 0.66±0.08, p=0.01). LDH/upper limit of normal, WHO performance status ≥1 and extranodal involvement ≥1 showed the highest performance for the clinical prediction model with a CV-AUC of 0.71±0.08. When combining radiomics features with clinical predictors, the highest performance was observed for the natural logarithms of MTV and SUVpeak, Dmaxbulk, WHO performance status ≥ 1 and age, with a CV-AUC of 0.77±0.07, which was significantly higher than the IPI model (p=0.003). Adding radiomics features to clinical predictors increased the positive predictive value with 15%, with more accurate selection of high-risk patients compared to the IPI model (progression at 2-year TTP: 28.3% vs 43.8%, respectively).
Conclusion
Combining quantitative radiomics features extracted from baseline 18F-FDG PET/CT scans with components of the IPI score significantly improved identification of patients at risk of relapse at baseline. Adding radiomics features can significantly increase the efficiency of clinical trials in poor prognosis patients.
Lugtenburg:Incyte: Honoraria; Celgene: Honoraria; Genmab: Honoraria; Genentech: Honoraria; Roche: Research Funding; Servier: Honoraria, Research Funding; Takeda: Honoraria, Research Funding. Zijlstra:Roche: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal